Epichlorohydrin is the main raw material for epoxy resin production, as well as important raw material in organic chemical industry and product in fine chemical industry.
Epichlorohydrin is the main raw material for epoxy resin production, as well as important raw material in organic chemical industry and product in fine chemical industry. The production of epichlorohydrin via the glycerol method primarily consists of two core sections:
● Chlorination Reaction Section: Raw material glycerol reacts with hydrogen chloride gas in the presence of a catalyst to produce the intermediate, dichloropropanol.
● Saponification/Cyclization Section: Dichloropropanol undergoes a saponification reaction with an alkali solution, removing hydrogen chloride to form epichlorohydrin via cyclization.
The entire process involves the recycling of materials and the treatment of by-products, representing a continuous, refined process.
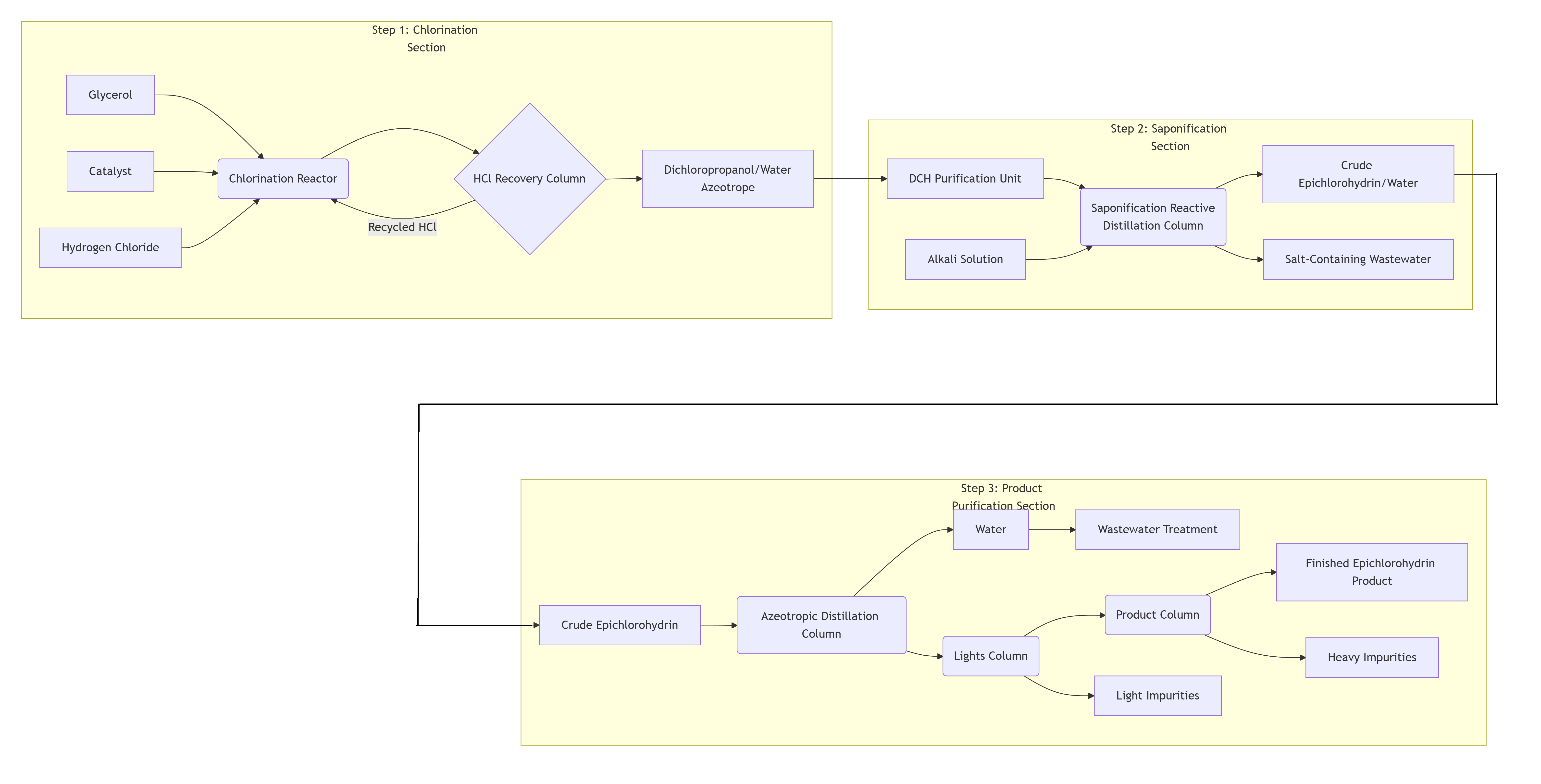
Three-Step Process Breakdown
Step 1: Chlorination Section – Generating the Intermediate
● Input Materials: Glycerol, Catalyst, Hydrogen Chloride gas.
● Core Unit: The Chlorination Reactor, where the catalytic chlorination reaction occurs.
● Key Step: The mixture from the reaction enters the HCl Recovery Column, where unreacted hydrogen chloride gas is separated and recycled back to the reactor, improving raw material utilization.
● Output Stream: The dichloropropanol/water azeotrope is produced and sent to the next section.
Step 2: Saponification/Cyclization Section – Forming the Product
● Input Materials: Dichloropropanol from the first section, Alkali solution.
● Core Unit: The Saponification Reactive Distillation Column. This is a key unit where reaction and separation occur simultaneously. Dichloropropanol reacts with the alkali, and the resulting epichlorohydrin is continuously vaporized due to its low boiling point.
● Output Streams:
Column Overhead: A mixture of crude epichlorohydrin and water is obtained.
Column Bottoms: Salt-containing wastewater is discharged and sent for treatment.
Step 3: Product Purification Section – Refining
This is a series of distillation columns designed to remove water and impurities from the crude product, yielding a high-purity final product.
● Azeotropic Distillation Column: Separates water from the crude product, yielding crude epichlorohydrin with very low water content.
● Lights Column: Removes light impurities with a lower boiling point than epichlorohydrin.
● Product Column: Operates under high vacuum to remove heavier, high-boiling point impurities.
● Final Product: High-purity finished epichlorohydrin is obtained as a side-stream or overhead product from the Product Column.
Technical Features
● Catalytic Chlorination Reaction: The core of this process is the gas-liquid phase reaction between glycerol and hydrogen chloride in the presence of a dedicated catalyst (e.g., carboxylic acids or esters) to directly produce dichloropropanol. The choice of catalyst is key to achieving high selectivity and conversion.
● Reactive Distillation Technology: In the saponification step, the reaction (cyclization of dichloropropanol) and the separation of the product (epichlorohydrin) occur simultaneously in the same unit—the reactive distillation column. This approach breaks chemical equilibrium limitations, improves reaction efficiency, and reduces energy consumption.
● HCl Recycling: Excess hydrogen chloride gas from the chlorination reaction is captured by a dedicated recovery system and recycled back to the reactor. This significantly improves atom economy and reduces raw material consumption and waste acid generation.
● Azeotropic Distillation for Purification: The process involves the separation of several azeotropes (e.g., dichloropropanol-water, epichlorohydrin-water). This requires a carefully designed sequence of azeotropic distillation steps to dehydrate streams and obtain high-purity products.
● Feedstock Flexibility: The process can accommodate crude glycerol derived from biodiesel production, which typically requires pre-treatment but reduces reliance on more expensive refined glycerol, enhancing process economics.
Key Advantages
● Exceptional Environmental Performance: This is its most prominent advantage. Compared to the traditional chlorohydrin process, it consumes no chlorine gas, reduces wastewater generation by approximately 90%, and produces wastewater free of persistent organic chlorides, making it easier to treat. It also avoids the production of large amounts of calcium chloride sludge.
● High Atom Economy: All three carbon atoms in the glycerol molecule are incorporated into the final product, and HCl utilization is very high, aligning with green chemistry principles.
● Relatively Short Process Flow: The direct production of dichloropropanol from glycerol involves fewer steps than the chlorohydrin process starting from propylene. The process flow is more compact, and capital investment is relatively lower.
● Utilization of Renewable Resources: Using biomass-derived glycerol as a feedstock reduces dependence on fossil-based raw materials (propylene), offering sustainability benefits.
● Milder Reaction Conditions: The main reactions proceed under moderate temperature and pressure, resulting in higher operational safety.
Product Specification
Epichlorohydrin (ECH)
Epichlorohydrin (ECH) product specification
Item |
Unit |
Specification |
Purity |
% wt |
>99.9 |
Water content |
ppm. Wt. |
<200 |
color |
APHA |
<15 |